Sunday, 11 August 2013
Wednesday, 24 July 2013
Wednesday, 26 June 2013
Vaginal hysterectomy first steps
Tuesday, 25 June 2013
Non PID causes of Ectopic pregnancy
Many patients ask me whenever I met up a client with a diagnosis of Ectopic pregnancy, what is the cause and why did this happen?
My usual answer use to be asymptomatic PID.
But my observations are that, eventhough PID may be the common cause of Ectopic pregnancy, but many took it by surprise. So that made me to keep a close eye on Non PID causes of Ectopic pregnancy.
If the Ectopic pregnancy is on the right side, then chronic appendicitis with adhesions should be thought of.
If the Ectopic pregnancy is on the left or right, then active endometriosis should be thought of.
My usual answer use to be asymptomatic PID.
But my observations are that, eventhough PID may be the common cause of Ectopic pregnancy, but many took it by surprise. So that made me to keep a close eye on Non PID causes of Ectopic pregnancy.
If the Ectopic pregnancy is on the right side, then chronic appendicitis with adhesions should be thought of.
If the Ectopic pregnancy is on the left or right, then active endometriosis should be thought of.
Tuesday, 18 June 2013
Vaginal hysterectomy -- Tips and tricks
The most important step in vaginal hysterectomy is safe dissection of the bladder and pouch of Douglas.
- The initial incision on cervix begins circumferentially at the reflection of the vaginal mucosa onto the cervix.
- The position and depth of this circumferential incision is very important because they determine access to appropriate planes that will lead to opening of Utero vescial fold of peritoneum and Pouch of Douglas peritoneum.
- Anteriorly appropriate location of the incision is at the site of the bladder reflection. The lower end of bladder reflection on the prolapsed cervix is indicated by a CREASE or SULCUS formed in the vaginal mucosa when the cervix is pushed slightly inward or back into the vagina. This can also be achieved by moving the cervix in and out of the vagina and at the same time try and identify the sulcus or the groove between the vagina and bladder reflection bulge. If this location is still not identified, one should make the incision low rather than high to avoid the potential bladder injury. Downward traction on the cervix and counter-traction by the retractors help to determine the appropriate depth of the incision. This incision should be continued down to the cervical stroma. Once the appropriate depth of the incision is reached, the vaginal tissue will fall away from the underlying cervical tissue because there is distinct plane between these two tissues.
Wednesday, 12 June 2013
Ovarian reserve assessment
Ovarian reserve
- Pattern of menstrual cycle assessment (regular /irregular)
- Antral follicle count
- Serum FSH (menstrual Day 2-5)
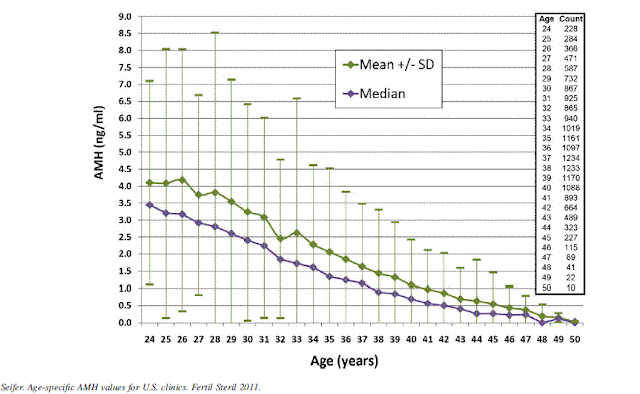
- AMH – Produced by the ovary and does not fluctuate. At menopause can become undetectable.
Fundamental basis : women with good ovarian reserve have sufficient production of ovarian hormones from small follicles early in the menstrual cycle to maintain FSH at a low level. In contrast, women with a reduced pool of follicles and oocytes have insufficient production of ovarian hormones to provide normal inhibition of pituitary secretion of FSH, so FSH rises early in the cycle. The constant relationship between the number of follicles and circulating antimullerian hormone exists only after the age of 25 years. The recognition
that antim€ullerian hormone (AMH) is produced by preantral and small antral follicles with serum concentrations reflecting both the number of these small growing follicles and the primordial pool (1–3) now allows the opportunity for the study of aspects of these
earlier stages and factors influencing development, as well as the investigation of their relationship with fertility.
Perform basal cycle day 3 FSH
A normal result is not useful in predicting fertility, but a highly abnormal result ( FSH >20 mIU/mL) suggests that pregnancy will not occur with treatment involving the woman's own oocytes. A day 3 FSH concentration and a value less than 10 mIU/mL suggestive of adequate ovarian reserve, and levels of 10 to 15 mIU/ml borderline
Perform cycle day3 Estradiol : a cycle day 3 estradiol level, although there are conflicting data as to whether it is predictive of ovarian reserve and the response to ovarian stimulation (a value <80 pg/mL suggestive of adequate ovarian reserve
Antral follicle count (AFC) : Number of antral follicles (defined as follicles measuring 2 to 10 mm in diameter). On transvaginal ultrasound, a low AFC ranging from 4 to 10 antral follicles between days two and four of a regular menstrual cycle suggests poor ovarian reserve. Although AFC is a good predictor of ovarian reserve and response, it is less predictive of oocyte quality, the ability to conceive with IVF.
Anti-müllerian hormone (AMH) — Anti-müllerian hormone (AMH) is expressed by the small (<8 mm) preantral and early antral follicles. The AMH level reflects the size of the primordial follicle pool. In adult women, AMH levels gradually decline as the primordial follicle pool declines with age. AMH is undetectable at menopause.
serum AMH levels correlate strongly to antral follicle count and are more accurate than age and other conventional serum markers (FSH, E2, inhibin B) in predicting preovulatory oocyte supply in response to ovulation induction. Relative to the conventional ovarian serum makers, AMH appears to vary significantly less throughout the menstrual cycle.
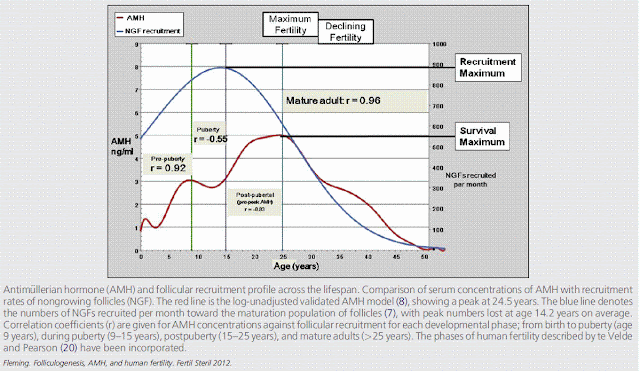
The circulating AMH also changes across the lifespan, with initial increases during childhood, then a distinct fluctuation around the time of puberty, followed by a secondary increase during the next decade to a maximum at around age 25 years. After the age 25, AMH undergoes a relentless decline to values below the levels of assay sensitivity between ages 40 and 45 years. This consistent decline in AMH from age 25 years is now firmly established.
After 25 years there is an uninterrupted and strong positive correlation betweenAMHand decreasing follicular recruitment as the pool of nongrowing follicles declines and eventually becomes exhausted at the menopause. It is this decline in follicular recruitment, albeit associated with
a maximal proportion of the available follicles achieving maturation, that results in a smaller number of follicles achieving the later stages of follicular development, which underlies the decline in AMH and explains the close relationship between AMH and egg yields seen in the IVF setting.
Antimullerian hormone is produced by growing follicles up to approximately 8 mm in diameter. Under normal circumstances much of the increase in circulating AMH arises after puberty.
PCOS and AMH : Women having PCO differ from those with normal ovaries by having slightly higher mean serum androgens or AMH levels. PCOs in normal women are not a morphological variant of normal ovaries but rather represent a functional entity that may be considered as a silent form of PCOS for which serum AMH could be the best marker. In conclusion, for the definition of PCOS, serum AMH appears as a sensitive and specific parameter that would probably be easier to reproduce from one to another centre than the follicle count, as the latter is highly dependent on the evolving quality of the machines and/or the operator skill. The threshold for AMH proposed to suggest the presence of Polycystic ovaries is 35 pmol/l (or 5 ng/ml).
Tuesday, 11 June 2013
Ulipristal acetate and Uterine fibroids
Ulipristal marketed as, a progesterone receptor antagonist (PRA). It acts by depriving uterine fibroids of growth stimulation due to progesterone.
The treatment consists of one tablet of 5 mg to be taken orally once daily for up to 3 months, and it should be started during the first week of a menstrual cycle. There are no data available on treatment with a duration longer than 3 months or on repeat courses of treatment. Therefore, treatment duration should not exceed 3 months.
The benefits with Esmya are its ability to reduce fibroid-related bleeding, anaemia and fibroid size. Ulispristal showed better efficacy compared to placebo (a dummy) at reducing bleeding and anaemia, but only moderated efficacy with regards to fibroid volume reduction.
The most common side effects are amenorrhea, endometrial thickening and hot flush.
The treatment consists of one tablet of 5 mg to be taken orally once daily for up to 3 months, and it should be started during the first week of a menstrual cycle. There are no data available on treatment with a duration longer than 3 months or on repeat courses of treatment. Therefore, treatment duration should not exceed 3 months.
The benefits with Esmya are its ability to reduce fibroid-related bleeding, anaemia and fibroid size. Ulispristal showed better efficacy compared to placebo (a dummy) at reducing bleeding and anaemia, but only moderated efficacy with regards to fibroid volume reduction.
The most common side effects are amenorrhea, endometrial thickening and hot flush.
Monday, 10 June 2013
Chronic Vaginal Discharge -- Management considerations
Vaginal discharge that is difficult
to treat: Management consdierations
It is normal and healthy for women
of reproductive age to have some degree of vaginal
discharge. The quantity and type of
cervical mucus changes during the menstrual cycle as a
result of hormonal fluctuations.
Prior to ovulation, estrogen levels increase, altering cervical
mucus from non-fertile (thick and
sticky) to fertile (clearer, wetter, stretchy and slippery). After
ovulation, estrogen levels fall and
progesterone levels increase; cervical mucus becomes
thick, sticky and hostile to sperm.
The vagina is colonised with commensal bacteria (normal vaginal flora). Rising
estrogen levels at puberty lead to colonisation with lactobacilli which
metabolise glycogen in the vaginal epithelium to produce lactic acid. Thus the
vaginal environment is acidic and normally has a pH≤4.5. Other commensal
bacteria include anaerobes, diphtheroids, coagulase-negative staphylococci and
α-hα-haemolytic streptococci. Some commensal organisms can cause a
change in discharge if they
‘overgrow’. These include Candida albicans, Staphylococcus
aureus and Group B streptococcus.
Commonest Causes of Altered
Vaginal Discharge in Women of Reproductive Age?
There are three common causes of
altered vaginal discharge in women of reproductive age:
1.
Infective (non-sexually transmitted)
a.
Bacterial vaginosis
b.
Candida
2.
Infective (sexually transmitted)
a.
Chlamydia trachomatis
b.
Neisseria gonorrhoeae
c.
Trichomonas vaginalis
d.
Herpes simplex virus
3.Non-infective
- Cervical polyps and ectropion
- Genital tract malignancy
- Allergic reactions.
4. Non-sexually transmitted
infections
Bacterial vaginosis
BV is the commonest cause of
abnormal vaginal discharge in women of reproductive age.2
Reported prevalence varies and may
be influenced by behavioural and/or
sociodemographic factors.3–5 It can
occur and remit spontaneously and is characterised by
an overgrowth of mixed anaerobic
organisms that replace normal lactobacilli, leading to an
increase in vaginal pH (>4.5). Gardnerella vaginalis is commonly found in
wommen with BV but the presence of Gardnerella alone is insufficient to
constitute a diagnosis of BV because it is a commensal organism in 30–40% of asymptomatic
women. Other organisms associated with BV include Prevotella species, Mycoplasma
hominis and Mobiluncus species.
BV is considered to be ‘sexually associated’
rather than truly ‘sexually transmitted’. There is some evidence that consistent
condom use may help to reduce BV prevalence,7,14–16 although one study
suggested this may only be in women
who were BV-negative at baseline.15
Vulvovaginal candidiasis (VVC)
VVC is common among women of
reproductive age. It is caused by overgrowth of yeasts;
C. albicans, in 70–90% of cases,
with non-albicans species such as C. glabrata in the
remainder. The presence of candida
in the vulvovaginal area does not necessarily require
treatment, unless symptomatic, as
between 10% and 20% of women will have vulvovaginal
colonisation.
Candidiasis occurs most commonly
when the vagina is exposed to estrogen, therefore it is more common during the
reproductive years and during pregnancy. An episode of VVC is
often precipitated by use of
antibiotics.Immunocompromised women20,21and women with
diabetes are predisposed to
candidiasis. VVC does not appear to be associated with
tampons, sanitary towels or panty
liners when they are used appropriately.24
As Vulvovaginal candida can be
found in non-sexually active individuals, it is not classed as an STI.
3.2 Sexually transmitted
infections
Chlamydia trachomatis
Chlamydia trachomatis, the most
common bacterial STI in the UK, is usually asymptomatic in
women (approximately 70%). However,
women may present with vaginal discharge due to
cervicitis, abnormal bleeding
(postcoital or intermenstrual) due to cervicitis or endometritis,
lower abdominal pain, dyspareunia
or dysuria.
Neisseria gonorrhoeae
Gonorrhoea is an STI caused by
Neisseria gonorrhoeae. Up to 50% of women will be
asymptomatic. Common symptoms may
include increased or altered vaginal discharge and
lower abdominal pain. It can also
be a rare cause of heavy menstrual, postcoital or
intermenstrual bleeding due to
cervicitis or endometritis.25
Trichomonas vaginalis
TV is a flagellated protozoan that
causes vaginitis. Women with TV commonly complain of
vaginal discharge and dysuria (due
to urethral infection).
TV is always sexually transmitted
and is a rarer condition than BV or VVC.
Herpes simplex
Women with cervicitis due to herpes
simplex virus infection may occasionally present with
vaginal discharge.
Other causes of vaginal
discharge
Other causes of vaginal discharge
include foreign bodies (e.g. retained tampons or
condoms), cervical ectopy or
polyps, genital tract malignancy, fistulae and allergic reactions.
Exclusion of infective and other
causes can help confirm that a vaginal discharge is
physiological.
There is some association between
methods of contraception and vaginal discharge. Women complaining of vaginal
discharge should be asked about current and past contraception.
Douching is the process of
intravaginal cleaning with a liquid solution. Some women use the
practice of douching as part of
their general hygiene or cultural practice. Data suggest that
douching changes vaginal flora and
may predispose women to BV, although not all
studies have reported this finding.
Overall, the evidence suggests that douching should be
discouraged as there are no proven
health benefits.
.
Women with cervical ectropion may
complain of increased physiological discharge. Ectopy is a
normal finding in women of
reproductive age but treatments such as acidic gel, silver nitrate
cauterisation, laser or cold
coagulation are occasionally used in a gynaecology setting for
symptomatic relief of vaginal
discharge or postcoital bleeding. There is a lack of robust
evidence for the effectiveness of
these treatments in reducing vaginal discharge. Cervical
pathology must be excluded prior to
treatment, and women’s should be informed of potential
risks of treatment and the fact
that discharge symptoms may initially worsen before there is
any improvement. evidence as to
whether the use of hormonal contraception increases the risk of VVC.
One study has suggested that the
progestogen-only injectable may reduce a woman’s susceptibility
to recurrent VVC, possibly because
of its anovulatory effect and relative hypoestrogenism.
Women using Combined hormonal
contraception who have recurrent VVC may wish to consider alternative
contraception but there is a lack of evidence to show whether there is any
benefit from switching to a lower dose combined preparation or a
progestogen-only method, other than the injectable.
The Cu-IUD has been identified as a
possible risk factor for acute or recurrent VVC, but there
is no consistent evidence of an
association. There is some evidence to demonstrate that
yeasts adhere to IUDs and the combined
vaginal ring (CVR). Combined vaginal ring users have been
reported as experiencing more
vaginal irritation and discharge compared with combined pill
users. However, a study of the
effect of CVR use on vaginal flora showed no increase in
numbers of inflammatory cells or
pathogenic bacteria.
Although cervical cytology slides
from levonorgestrel-releasing intrauterine system (LNG-IUS)
users have shown increased presence
of candida with time from insertion, rates of
symptomatic infection did not
change significantly.
Bacterial vaginosis
Oral combined contraception and
condoms have been associated with a reduced risk of
BV, whilst BV is more common in
users of the Cu-IUD. The association between BV and
use of the LNG-IUS is unclear. The
progestogen-only implant and injectable may be
associated with a decreased risk of
BV.Women using CHC who experience recurrent VVC may wish to consider switching
to an alternative method of contraception. Women with a Cu-IUD who experience
recurrent BV may wish to consider switching to an alternative method of
contraception.
8 Personal Hygiene and Vaginal
Discharge
Personal hygiene measures can be
advised for women who are prone to vaginal discharge
and/or pruritis (e.g. regular
changing of sanitary protection, avoidance of douching and of
potentially irritant chemicals in
toiletries, antiseptics, wipes, so-called ‘feminine hygiene’
products, washing powders, fabric
dyes, and so on). RCOG guidance contains patient
information on general care of the
vulval skin, including use of emollients and soap substitutes
which prevent dryness and loss of
the skin’s natural barrier functions.Women experiencing vaginal discharge can
be advised to avoid douching and local irritants as part of general management.
Health professionals should be
aware that the most common causes of altered vaginal
discharge are physiological, BV and
candida, but STIs and non-infective causes must be
considered. Table 1 Summary of
signs and symptoms of infective causes of vaginal discharge
Sign/symptom Bacterial vaginosis
Candida Trichomoniasis
Discharge Thin Thick white Scanty
to profuse
Odour Offensive/fishy Non-offensive
Offensive
Itch None Vulval itch Vulval itch
Other possible symptoms Soreness
Dysuria
Superficial dyspareunia Lower
abdominal pain
Dysuria
Visible signs Discharge coating the
Normal findings Frothy yellow discharge
vagina and vestibule or Vulvitis
Vaginitis
No vulval inflammation Vulval
erythema Cervicitis
Oedema ‘Strawberry cervix’
(ectocervix
Fissuring sometimes resembles the
surface of
Satellite lesions a strawberry)
Point-of-care test: vaginal pH
>4.5 ≤4.5 >4.5
Saturday, 8 June 2013
Abnormal Uterine Bleeding
Unscheduled vaginal bleeding—bleeding that occurs outside the normal menstrual period or the regular withdrawal bleed associated with the combined oral contraceptive pill—is a common reason for women of reproductive age to attend specialist care.It is also referred to as intermenstrual bleeding
Friday, 7 June 2013
No touch technique of Hysteroscopy
First do vaginoscopy.
Use betadine iodine to clean in the beginning
Close the vaginal opening with the other hand so that you create a water seal.
Ask the Anaesthesist for a head down position, so that water is held in the vagina longer.
In the beginning one can go up to the posterior fornix and then withdraw slightly to see a smooth surface of posterior wall of cervix.
In a parous woman, external os appears like a fish mouth.
If Betadine used to cleanse the vagina, usually some betadine will stain the cervical glands and this will be very obvious and will help to enter the cervical os.
There is a small possibility that the internal os may appear similar to one tubal ostia, so one may infact say this as a unicornuate uterus. Before concluding this one can spend little bit more time and hydro-distend the uterine cavity and a gentle nudge will negotiate the hysteroscope into the uterus.
Video
Use betadine iodine to clean in the beginning
Close the vaginal opening with the other hand so that you create a water seal.
Ask the Anaesthesist for a head down position, so that water is held in the vagina longer.
In the beginning one can go up to the posterior fornix and then withdraw slightly to see a smooth surface of posterior wall of cervix.
In a parous woman, external os appears like a fish mouth.
If Betadine used to cleanse the vagina, usually some betadine will stain the cervical glands and this will be very obvious and will help to enter the cervical os.
There is a small possibility that the internal os may appear similar to one tubal ostia, so one may infact say this as a unicornuate uterus. Before concluding this one can spend little bit more time and hydro-distend the uterine cavity and a gentle nudge will negotiate the hysteroscope into the uterus.
Video
Video 2
Monday, 3 June 2013
Uterine polyps :Management considerations
In both pre and postmenopausal women, endometrial polyps lose their apoptotic regulation and overexpress estrogen and progesterone receptors, thus avoiding the usual control mechanisms.
Endometrial polyps are present in approximately one-quarter of symptomatic pre and postmenopausal women.
Half of the premenopausal women present with menorrhagia; other presentations include postmenopausal bleeding, prolapse through the cervical ostium, abnormal vaginal discharge and breakthrough bleeding during hormonal therapy.
Increased incidence of endometrial polyps in women on hormone replacement therapy (HRT) and tamoxifen (8-36%), which acts as a selective receptor modulator and estrogen agonist on the endometrium. The influence on endometrial polyps seems to be through estrogen, on which endometrial polyps depend. However, endometrial polyp formation appears to be related to the type and dosage of the estrogen and progestogen in HRT; in particular, a progestogen with high anti-estrogenic activity may have an important role in preventing the development of endometrial polyps.
Diabetes, hypertension and obesity are independent risk factors for the development of endometrial polyps. Predictors of malignancy or premalignancy in endometrial polyps : a size of >10 mm postmenopausal status abnormal uterine bleeding a polyp diameter A polyp of >18 mm in asymptomatic women increased the risk of malignancy there is a higher incidence of concurrent endometrial hyperplasia with endometrial polyps,13, 14 especially in women on hormone replacement.
Hysteroscopic markers for malignant endometrial polyps include surface irregularities such as necrosis, vascular irregularities and whitish thickened areas, which are indications for obtaining a histological diagnosis.
Fertility and endometrial polyps Large or multiple endometrial polyps can contribute to infertility and increase the risk of miscarriage.Hysteroscopic polypectomy will improve the rate of spontaneous conception regardless of size or number of polyps, which may be due to the normalisation of endometrial implantation fayctors
Treatment of endometrial polyps: The risk of malignant transformation of endometrial polyps is low, but they should be removed when detected, as excision allows for both histological diagnosis and effective treatment of abnormal uterine bleeding patterns and excessive menstrual loss; in addition, endometrial polyps in postmenopausal women are more likely to be malignant when symptomatic
What to do with asymptomatic and incidental finding of endometrial polyps?
Asymptomatic and incidental endometrial polyps should be treatedause for endometrial polyps In both pre and postmenopausal women, endometrial polyps lose their apoptotic regulation and overexpress estrogen and progesterone receptors, thus avoiding the usual control mechanisms.
Endometrial polyps are present in approximately one-quarter of symptomatic pre and postmenopausal women. Half of the premenopausal women present with menorrhagia; other presentations include postmenopausal bleeding, prolapse through the cervical ostium, abnormal vaginal discharge and breakthrough bleeding during hormonal therapy. Increased incidence of endometrial polyps in women on hormone replacement therapy (HRT) and tamoxifen (8-36%), which acts as a selective receptor modulator and estrogen agonist on the endometrium. The influence on endometrial polyps seems to be through estrogen, on which endometrial polyps depend. However, endometrial polyp formation appears to be related to the type and dosage of the estrogen and progestogen in HRT; in particular, a progestogen with high anti-estrogenic activity may have an important role in preventing the development of endometrial polyps. Diabetes, hypertension and obesity were independent risk factors for the development of endometrial polyps. Predictors of malignancy or premalignancy in endometrial polyps : a size of >10 mm postmenopausal status abnormal uterine bleeding a polyp diameter A polyp of >18 mm in asymptomatic women increased the risk of malignancy there is a higher incidence of concurrent endometrial hyperplasia with endometrial polyps,13, 14 especially in women on hormone replacement. Hysteroscopic markers for malignant endometrial polyps include surface irregularities such as necrosis, vascular irregularities and whitish thickened areas, which are indications for obtaining a histological diagnosis. Fertility and endometrial polyps Large or multiple endometrial polyps can contribute to infertility and increase the risk of miscarriage.Hysteroscopic polypectomy will improve the rate of spontaneous conception regardless of size or number of polyps, which may be due to the normalisation of endometrial implantation fayctors Treatment of endometrial polyps: The risk of malignant transformation of endometrial polyps is low, but they should be removed when detected, as excision allows for both histological diagnosis and effective treatment of abnormal uterine bleeding patterns and excessive menstrual loss; in addition, endometrial polyps in postmenopausal women are more likely to be malignant when symptomatic What to do with asymptomatic and incidental finding of endometrial polyps? Asymptomatic and incidental endometrial polyps should be treated. Algorithm for management of endometrial polyps. Whether to avulse blindly or resect hysteroscopically? There is good direct and circumstantial evidence that hysteroscopic resection of endometrial polyps under vision is safe, simple and superior to blind techniques: There is a possibility that malignant cells can be missed if one uses blind technique of avulsion Hysteroscopic resection avoids excessive cervical dilatation, which is when uterine perforation and creation of a false passage usually occur With the blind avulsion technique, recurrence rate of 15% and none with resection technique. Polyps>2 cm require piecemeal removal, a longer operating time and multiple instrument passes through the cervix. In those cases removal under general Anaesthesia is advisable, but small-diameter hysteroscopic morcellators can also be considered.. Algorithm for management of endometrial polyps. Whether to avulse blindly or resect hysteroscopically? There is good direct and circumstantial evidence that hysteroscopic resection of endometrial polyps under vision is safe, simple and superior to blind techniques: There is a possibility that malignant cells can be missed if one uses blind technique of avulsion Hysteroscopic resection avoids excessive cervical dilatation, which is when uterine perforation and creation of a false passage usually occur With the blind avulsion technique, recurrence rate of 15% and none with resection technique. Polyps>2 cm require piecemeal removal, a longer operating time and multiple instrument passes through the cervix. In those cases removal under general Anaesthesia is advisable, but small-diameter hysteroscopic morcellators can also be considered.
Endometrial polyps are present in approximately one-quarter of symptomatic pre and postmenopausal women.
Half of the premenopausal women present with menorrhagia; other presentations include postmenopausal bleeding, prolapse through the cervical ostium, abnormal vaginal discharge and breakthrough bleeding during hormonal therapy.
Increased incidence of endometrial polyps in women on hormone replacement therapy (HRT) and tamoxifen (8-36%), which acts as a selective receptor modulator and estrogen agonist on the endometrium. The influence on endometrial polyps seems to be through estrogen, on which endometrial polyps depend. However, endometrial polyp formation appears to be related to the type and dosage of the estrogen and progestogen in HRT; in particular, a progestogen with high anti-estrogenic activity may have an important role in preventing the development of endometrial polyps.
Diabetes, hypertension and obesity are independent risk factors for the development of endometrial polyps. Predictors of malignancy or premalignancy in endometrial polyps : a size of >10 mm postmenopausal status abnormal uterine bleeding a polyp diameter A polyp of >18 mm in asymptomatic women increased the risk of malignancy there is a higher incidence of concurrent endometrial hyperplasia with endometrial polyps,13, 14 especially in women on hormone replacement.
Hysteroscopic markers for malignant endometrial polyps include surface irregularities such as necrosis, vascular irregularities and whitish thickened areas, which are indications for obtaining a histological diagnosis.
Fertility and endometrial polyps Large or multiple endometrial polyps can contribute to infertility and increase the risk of miscarriage.Hysteroscopic polypectomy will improve the rate of spontaneous conception regardless of size or number of polyps, which may be due to the normalisation of endometrial implantation fayctors
Treatment of endometrial polyps: The risk of malignant transformation of endometrial polyps is low, but they should be removed when detected, as excision allows for both histological diagnosis and effective treatment of abnormal uterine bleeding patterns and excessive menstrual loss; in addition, endometrial polyps in postmenopausal women are more likely to be malignant when symptomatic
What to do with asymptomatic and incidental finding of endometrial polyps?
Asymptomatic and incidental endometrial polyps should be treatedause for endometrial polyps In both pre and postmenopausal women, endometrial polyps lose their apoptotic regulation and overexpress estrogen and progesterone receptors, thus avoiding the usual control mechanisms.
Endometrial polyps are present in approximately one-quarter of symptomatic pre and postmenopausal women. Half of the premenopausal women present with menorrhagia; other presentations include postmenopausal bleeding, prolapse through the cervical ostium, abnormal vaginal discharge and breakthrough bleeding during hormonal therapy. Increased incidence of endometrial polyps in women on hormone replacement therapy (HRT) and tamoxifen (8-36%), which acts as a selective receptor modulator and estrogen agonist on the endometrium. The influence on endometrial polyps seems to be through estrogen, on which endometrial polyps depend. However, endometrial polyp formation appears to be related to the type and dosage of the estrogen and progestogen in HRT; in particular, a progestogen with high anti-estrogenic activity may have an important role in preventing the development of endometrial polyps. Diabetes, hypertension and obesity were independent risk factors for the development of endometrial polyps. Predictors of malignancy or premalignancy in endometrial polyps : a size of >10 mm postmenopausal status abnormal uterine bleeding a polyp diameter A polyp of >18 mm in asymptomatic women increased the risk of malignancy there is a higher incidence of concurrent endometrial hyperplasia with endometrial polyps,13, 14 especially in women on hormone replacement. Hysteroscopic markers for malignant endometrial polyps include surface irregularities such as necrosis, vascular irregularities and whitish thickened areas, which are indications for obtaining a histological diagnosis. Fertility and endometrial polyps Large or multiple endometrial polyps can contribute to infertility and increase the risk of miscarriage.Hysteroscopic polypectomy will improve the rate of spontaneous conception regardless of size or number of polyps, which may be due to the normalisation of endometrial implantation fayctors Treatment of endometrial polyps: The risk of malignant transformation of endometrial polyps is low, but they should be removed when detected, as excision allows for both histological diagnosis and effective treatment of abnormal uterine bleeding patterns and excessive menstrual loss; in addition, endometrial polyps in postmenopausal women are more likely to be malignant when symptomatic What to do with asymptomatic and incidental finding of endometrial polyps? Asymptomatic and incidental endometrial polyps should be treated. Algorithm for management of endometrial polyps. Whether to avulse blindly or resect hysteroscopically? There is good direct and circumstantial evidence that hysteroscopic resection of endometrial polyps under vision is safe, simple and superior to blind techniques: There is a possibility that malignant cells can be missed if one uses blind technique of avulsion Hysteroscopic resection avoids excessive cervical dilatation, which is when uterine perforation and creation of a false passage usually occur With the blind avulsion technique, recurrence rate of 15% and none with resection technique. Polyps>2 cm require piecemeal removal, a longer operating time and multiple instrument passes through the cervix. In those cases removal under general Anaesthesia is advisable, but small-diameter hysteroscopic morcellators can also be considered.. Algorithm for management of endometrial polyps. Whether to avulse blindly or resect hysteroscopically? There is good direct and circumstantial evidence that hysteroscopic resection of endometrial polyps under vision is safe, simple and superior to blind techniques: There is a possibility that malignant cells can be missed if one uses blind technique of avulsion Hysteroscopic resection avoids excessive cervical dilatation, which is when uterine perforation and creation of a false passage usually occur With the blind avulsion technique, recurrence rate of 15% and none with resection technique. Polyps>2 cm require piecemeal removal, a longer operating time and multiple instrument passes through the cervix. In those cases removal under general Anaesthesia is advisable, but small-diameter hysteroscopic morcellators can also be considered.
Subscribe to:
Comments (Atom)